Prof. Satyajit Mayor
Welcome to Prof Satyajit Mayor's Lab at National Centre for Biological Sciences, Bangalore, India
Mechanisms of membrane organization and endocytosis
How a eukaryotic cell constructs signaling complexes on the plasma membrane and regulates membrane trafficking is the primary focus of our laboratory.
The plasma membrane does not merely separate the outside from the inside of a cell but also mediates the bilateral communication. To understand how eukaryotic cells respond and react to their environment, we study how a cell can regulate the local organization of its membrane constituents, while the membrane itself behaves like a fluid matrix. New insights from a variety of studies, including that from our laboratory, show that the local chemistry of these 2D plasma membranes is finely tuned and far from an equilibrium mixture. We are providing a new framework wherein the cell membrane behaves as an active composite, with the underlying dynamic cortical actin filaments controlling the local composition of membranes. There are numerous offshoots from such an understanding of membrane organization. Among them, we now seek to explain how cells can construct signalling complexes and sort membrane constituents, in response to their environment. The cell membrane also is the site for the assembling endocytic machinery, in response to a number of extrinsic and intrinsic cues. To broaden our understanding of membrane homeostasis, we also study endocytic mechanism, in particular a class of non-canonical endocytic pathway that functions in the absence of both clathrin and dynamin.
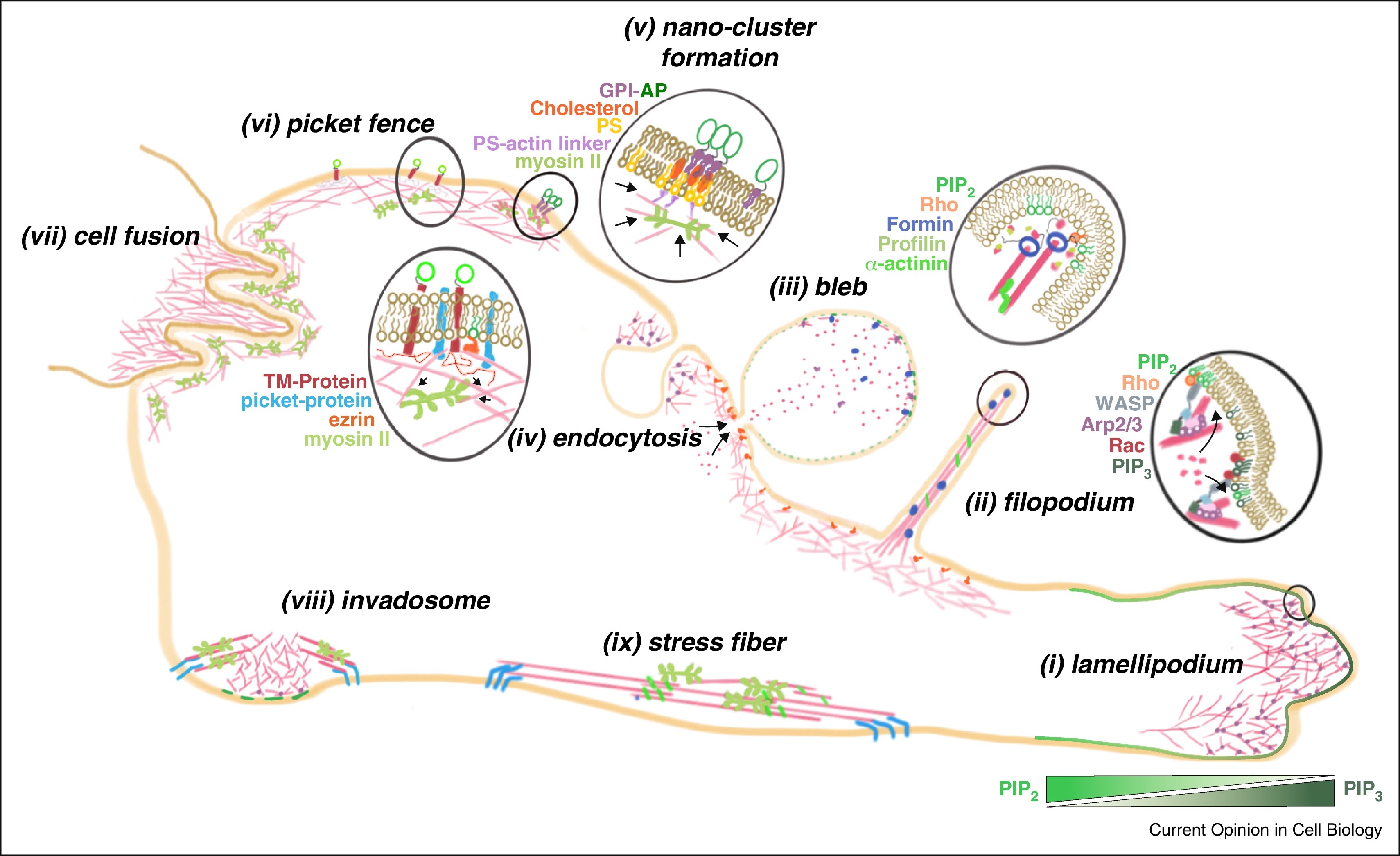
(Koester DV, Mayor S. Cortical actin and the plasma membrane : inextricably intertwined. Curr Opin Cell Biol. 2016)



